

How WAYRILZ was studied
WAYRILZ was studied in over 200 people with chronic ITP
The clinical study focused on adults with persistent or chronic immune thrombocytopenia who didn’t respond well to or couldn’t tolerate previous treatments. It compared 133 people who took WAYRILZ to 69 people who did not for a total of 24 weeks (or 6 months).
What does it mean to be a "Responder"?

Responders were patients whose platelet counts improved by month 3 of the study.
This meant reaching a platelet response (at least 50,000 or between 30,000 and 50,000 and doubling from their starting level) without needing rescue treatments.
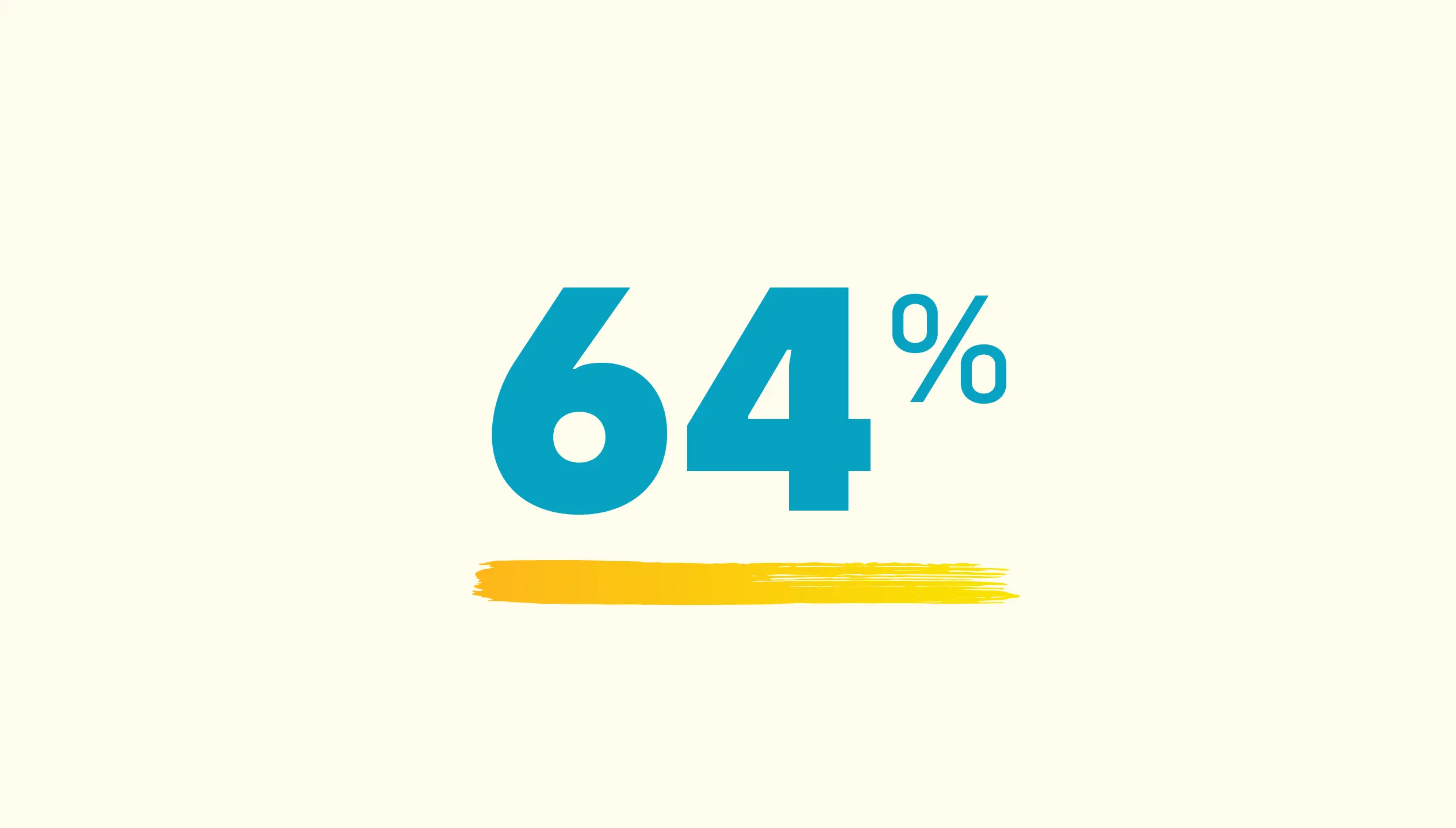
64% of people taking WAYRILZ were responders compared to 32% of people not taking WAYRILZ.
These patients continued to receive treatment for the full 6-month study.
Long-lasting platelet control with WAYRILZ
WAYRILZ improves and sustains platelet counts
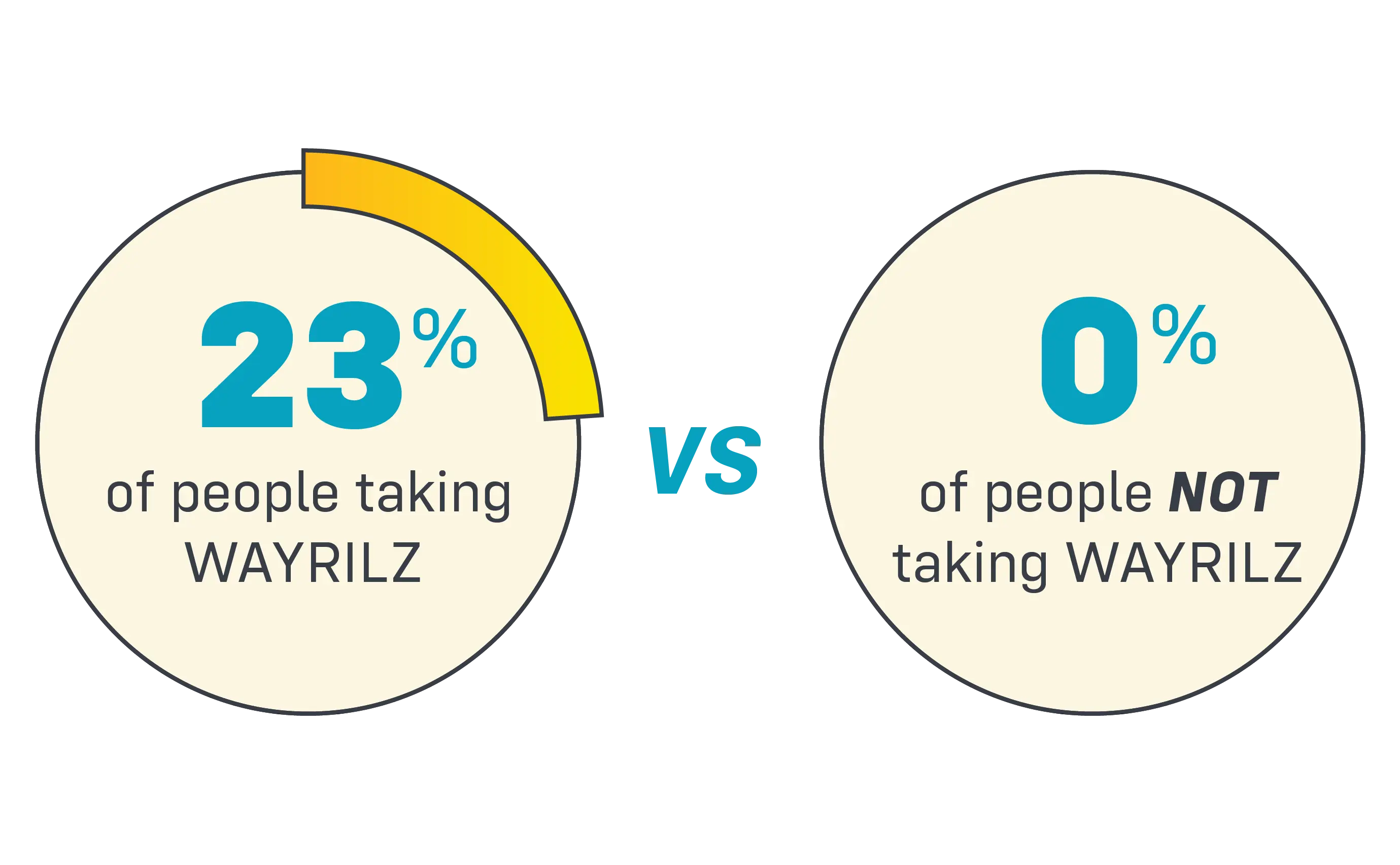
By month 6 in the clinical study, 23% of people taking WAYRILZ reached and maintained target platelet counts (at least 50,000) compared to 0% of people not taking WAYRILZ.
During the last 12 weeks of the study, people reached a platelet level of 50,000 or higher more than 5 times when measured weekly, without rescue therapy, including at least twice in the final 6 weeks.
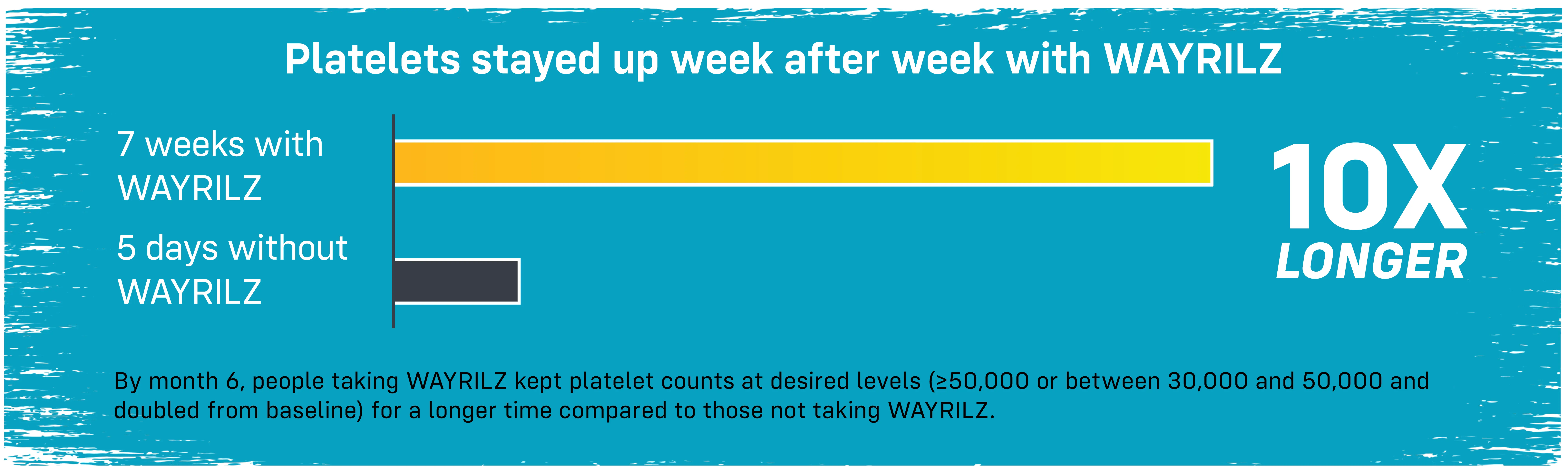
Platelet counts rose quickly
People taking WAYRILZ (133 people) reached target platelet levels in 36 days. Those not taking WAYRILZ (69 people) took longer or didn’t reach target levels by the end of the study.† Responders taking WAYRILZ (85 people) reached target platelet levels typically around 2 weeks.
†During the study, these people reached a platelet level of 50,000 or higher or between 30,000 and less than 50,000 and at least doubled from starting level, without rescue therapy.

People taking WAYRILZ needed fewer rescue treatments
During the study, medicines like IVIg, or intravenous immunoglobulin, were given only if needed. 58% of people who did not take WAYRILZ needed additional treatment compared to only 33% of people taking WAYRILZ.
People taking WAYRILZ reported impact on quality of life
How impact on quality of life was evaluated
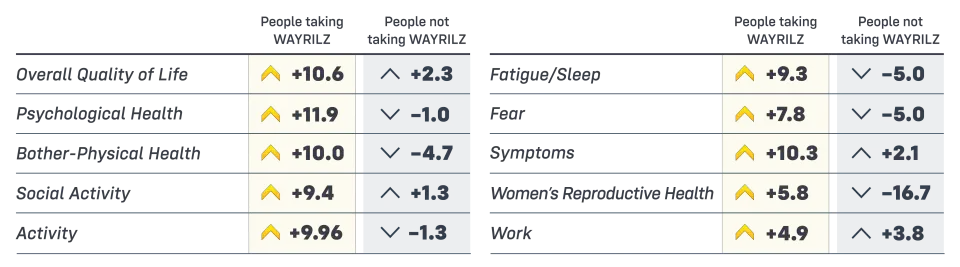
Prior to the start of the study, patients completed a survey called the ITP-PAQ that looked at how ITP affected different parts of their lives. At month 6, patients took the ITP-PAQ survey again. Each measurement was reported with a score ranging from 0 to 100, with a higher score indicating improving (^) and lower score indicating worsening (⌄).
For example, people taking WAYRILZ saw an improvement in overall quality of life by 10.6 points vs 2.3 points for those not taking WAYRILZ.
The results of these analyses are descriptive and not statistically tested to determine a difference between treatment groups.
WAYRILZ is a prescription medicine that is used to treat adults with persistent or chronic immune thrombocytopenia (ITP) who received a prior treatment that did not work well enough.
It is not known if WAYRILZ is safe and effective in children.
What should I tell my healthcare provider before taking WAYRILZ?
Tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems
- have kidney problems
- are pregnant or plan to become pregnant. WAYRILZ may harm your unborn baby. If you are able to have a baby, your healthcare provider will do a pregnancy test before starting treatment with WAYRILZ. Females who are able to become pregnant should use an effective birth control during treatment with WAYRILZ and for 1 week after the last dose.
- are breastfeeding or plan to breastfeed. Do not breastfeed during treatment with WAYRILZ and for at least 1 week after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over‑the‑counter medicines, vitamins, and herbal supplements. Taking WAYRILZ with certain other medicines may affect how WAYRILZ works and can cause side effects. WAYRILZ may also affect how other medicines work.
What should I avoid while taking WAYRILZ?
You should avoid grapefruit, starfruit and products that have these fruits, and Seville oranges (often used in marmalades) during treatment with WAYRILZ. These products may increase the amount of WAYRILZ in your blood, which increases the risk of side effects of WAYRILZ.
What are the possible side effects of WAYRILZ?
WAYRILZ may cause serious side effects, including:
- Serious infections. WAYRILZ can increase the risk of infections, including serious infections that can lead to death. Your healthcare provider will check you for signs and symptoms of infection during your treatment with WAYRILZ. Tell your healthcare provider right away if you get any signs or symptoms of infection, including fever, chills, or flu-like symptoms.
- Liver problems including Drug-Induced Liver Injury (DILI). Liver problems, which may be severe, life-threatening, or lead to death have happened in people treated with Bruton’s tyrosine kinase (BTK) inhibitors. Your healthcare provider will do blood tests to check your liver before and as necessary during treatment with WAYRILZ. Tell your healthcare provider right away if you have any signs or symptoms of liver problems, including stomach-area (abdominal) pain or discomfort, dark or “tea-colored” urine, or yellowing of the skin or the white part of your eyes.
The most common side effects of WAYRILZ include diarrhea, nausea, headache, stomach area (abdominal) pain, and COVID-19.
These are not all of the possible side effects of WAYRILZ.
Please see full Prescribing Information, including Patient Information.